Dalton Model of the Atom Is Best Described as
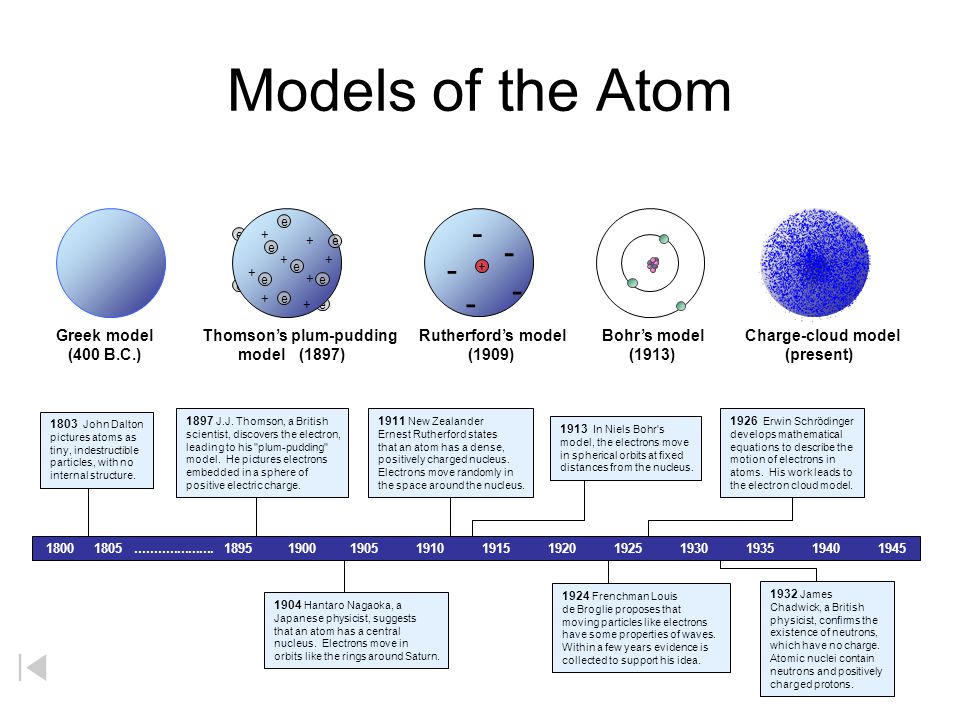
The Plum Pudding Model of the atom. Similar to Saturn with electrons of varying.

Development Of The Atom Chemistry Classroom Chemistry Lessons Chemistry Class
An electron is a particle with what.

. The current model of an atom is best described by the Solar System. All matter is composed of tiny particles called atoms. While all atoms of an element were identical different elements had atoms of differing size and mass.
A solid sphere c. The first scientist to develop a model of the atom which he described as looking like plum pudding a. Daltons model of an atom is best described as what.
Compare wave and particle models of light. The description of the structure of the atom is called the ___. The Rutherford Model of the atom.
Almost all the mass of an atom is located in its what. These had been known for about 50 years to be emitted in vacuum by a heated filament a cathode in a strong electric field. Daltons model of atom described the atom as the smallest indivisible particle of matter.
A negative charge. Models of the Atom Particle model of matter. A depiction of the atomic structure of the helium atom.
Is shows electrons floating freely in a positive space. Atoms are indivisible and indestructible. Model 3 Correct Answer.
The first scientist to develop a model of the atom which he described as looking like plum pudding a. A large lump of chocolate chip cookie. Francis Bacon performed human dissections and published a book filled with detailed drawings of human organs bones and muscles.
Democritus Democritus inferred that all matter is composed of small indestructible particles he named atomos. Democrituss model of the atom. Atoms were regarded as the smallest possible particles until JJ.
Daltons atomic theory proposed that all matter was composed of atoms indivisible and indestructible building blocks. Previously an atom was defined as the smallest part of an element that maintains the identity of that element. Ernest Rutherford ____ 9.
A plum pudding d. Daltons atomic theory also stated that all compounds were composed of combinations of these atoms in defined ratios. This was not a completely new concept as the ancient Greeks notably Democritus had proposed that all matter is composed of small indivisible cannot be divided objects.
Who provided the first evidence that atoms contain subatomic particles. Which item best represents Daltons mental. This was not a completely new concept as the ancient Greeks notably Democritus had proposed that all matter is composed of small indivisible cannot be divided objects.
Daltons model of the atom ESAAO John Dalton proposed that all matter is composed of very small things which he called atoms. The modern atomic theory proposed about 1803 by the English chemist John Dalton Figure 15. 3 Compounds are formed by a combination of two or more different kinds of atoms.
This became the basis as Daltons Law aka. Model 3 represents Daltons model of the atom which described atoms as tiny indestructible particles. Daltons design of the atom had different materials background and idea.
Select the best answer for each of the following questions. Daltons model of an atom is best described as a. Ouch I jammed my toe.
Nothing smaller than the atom. The word atom comes from the Greek atomos which means. This model was developed after JJ.
Those of higher energy are on the outside surface. Which best describes Francis Bacons contribution to the Scientific Revolution. What is different in Daltons model of the atom to todays model of the atom.
It was commonly described as a Solid sphere. The planets describe the size of the nucleus of the atom compared to the whole atom. Models of the Atom Through Time.
What phenomena can only be explained by the particle model. The Solar System Model of the atom. The scientist whose alpha-particle scattering experiment led him to conclude that the nucleus of an atom contains a dense center of positive charge is.
The combination of the types of particles in a substance determines what kind of matter it is. 4 is a fundamental concept that states that all elements are composed of atoms. Thompson discovered electrons a particle smaller than an atom.
1 All matter is made of atoms. Thomson discovered the electron. He was examining cathode rays.
Dough with electrons as the chocolate chips. Atom denoted as H 2 O rather than one hydrogen atom attached to one oxygen atom or HO as Dalton wrongly believed. Daltons model of the atom ESAAO John Dalton proposed that all matter is composed of very small things which he called atoms.
Energies in a single circular ring orbiting. 2 All atoms of a given element are identical in mass and properties. Daltons model of an atom is best described as.

What Are The 5 Postulates Of Dalton S Atomic Theory Atomic Theory Chemistry Lessons Study Chemistry

John Daltons Atom Theory Model Did Include The Charges Atomic Theory Dalton Atomic Model Atom Dalton

Dalton Atomic Model Rutherford Model Atom Model

Unit 1 1 2a Historical Development Of The Model Of Atomic Theory From Dalton To Chadwick Chemistry Teacher Chemistry Education Chemistry Lessons

Illustration Of Chemistry Atomic Models Scientific Theory Of The Nature Of Matter Ad Spon Atomic Models Illus Atom Model Atom Drawing Scientific Poster

Daltons Atomic Theory Easy Science Atomic Theory John Dalton Atomic Theory Chemical Changes

Atomic Models Downloadable Poster Science Print Chemistry Lessons Teaching Chemistry Chemistry Education

Scientific Explorer May 2012 Dalton Atomic Model Atom Model Dalton Model

Atomic Models And Their Innovations Atomicmodel Atomicmodels Daltonmodel Thomsonmodel Rutherfordmodel Bohrmod Chemistry Lessons Atom Chemistry Classroom

Bond With James Photo Of The Day Next Top Atomic Model Chemistry Lessons Teaching Chemistry Chemistry Classroom

Scientific Explorer May 2012 Dalton Atomic Model Atom Model Dalton Model

Dalton Atomic Model Rutherford Model Atom Model

Dalton S Atomic Theory Atomic Theory Atom Model John Dalton Atomic Theory

Class 9 Science Notes Chapter 4 Structure Of The Atom Science Notes Chemistry Classroom Biology Notes




Comments
Post a Comment